West Nile virus
| West Nile Virus | |
|---|---|
 |
|
| Virus classification | |
| Group: | Group IV ((+)ssRNA) |
| Family: | Flaviviridae |
| Genus: | Flavivirus |
| Species: | West Nile virus |
| West Nile Fever | |
|---|---|
| Classification and external resources | |
| ICD-10 | A92.3 |
| ICD-9 | 066.3 |
| DiseasesDB | 30025 |
| MeSH | D014901 |
West Nile virus (WNV) is a virus of the family Flaviviridae. Part of the Japanese encephalitis (JE) antigenic complex of viruses, it is found in both tropical and temperate regions. It mainly infects birds, but is known to infect humans, horses, dogs, cats, bats, chipmunks, skunks, squirrels, and domestic rabbits. The main route of human infection is through the bite of an infected mosquito. Approximately 90% of West Nile Virus infections in humans are without any symptoms.[1]
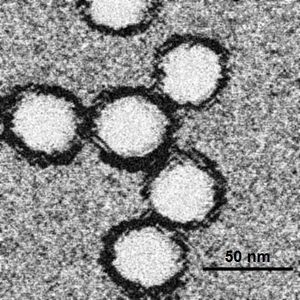
Image reconstructions and cryoelectron microscopy reveal a 45–50 nm virion covered with a relatively smooth protein surface. This structure is similar to the dengue fever virus; both belong to the genus Flavivirus within the family Flaviviridae. The genetic material of WNV is a positive-sense, single strand of RNA, which is between 11,000 and 12,000 nucleotides long; these genes encode seven non-structural proteins and three structural proteins. The RNA strand is held within a nucleocapsid formed from 12 kDa protein blocks; the capsid is contained within a host-derived membrane altered by two viral glycoproteins.
Contents |
Symptoms
WNV has three different effects on humans. The first is an asymptomatic infection; the second is a mild febrile syndrome termed West Nile Fever;[2] the third is a neuroinvasive disease termed West Nile meningitis or encephalitis.[3] The population proportion of these three states is roughly 110:30:1.[4]
The second, febrile stage has an incubation period of 2 to 8 days followed by fever, headache, chills, diaphoresis (excessive sweating), weakness, lymphadenopathy (swollen lymph nodes), drowsiness, pain in the joints and symptoms like those of influenza or the flu. Occasionally there is a short-lived truncal rash and some patients experience gastrointestinal symptoms including nausea, vomiting, loss of appetite, or diarrhea. Symptoms are generally resolved within 7 to 10 days, although fatigue can persist for some weeks and lymphadenopathy up to two months.
The more dangerous encephalitis is characterized by similar early symptoms but also a decreased level of consciousness, sometimes approaching near-coma. Deep tendon reflexes are hyperactive at first, later diminished. There are also extrapyramidal disorders. Recovery is marked by a long convalescence with fatigue.
More recent outbreaks have resulted in a deeper study of the disease and other, rarer, outcomes have been identified. The spinal cord may be infected, marked by anterior myelitis with or without encephalitis.[5] WNV-associated Guillain-Barré syndrome has been identified[6] and other rare effects include multifocal chorioretinitis (which has 100% specificity for identifying WNV infection in patients with possible WNV encephalitis),[7] hepatitis, myocarditis, nephritis, pancreatitis, and splenomegaly.[8][9][10]
Mortality rate
There is no way for the government to accurately measure the number of worldwide cases at this time. However, the United States keeps records of West Nile infection cases. In 2009, there were 663 cases. 335 of these cases were Encephalitis or Meningitis infections, a reaction to the virus that approximately 1 in 150 people who get the virus will show. 302 cases were filed for West Nile fever, the most likely symptom of the virus. 26 cases were unspecified. The state of Texas had the most cases, with 104 total. The total mortality rate for 2009 was 30 deaths of the 663 reported serious cases. That is a 4.5% casualty rate, but only of the severe infections. Approximately 80% of cases have no symptoms, and therefore the total casualty rate would be less than 1% of total infections in the U.S. This data and earlier years data is available from the CDC here:[11]
Transmission and susceptibility
Transmission

The virus is transmitted through mosquito vectors, which bite and infect birds. The birds are amplifying hosts, developing sufficient viral levels to transmit the infection to other biting mosquitoes which go on to infect other birds (in the Western hemisphere the American robin and the American crow are the most common carriers) and also humans. The infected mosquito species vary according to geographical area; in the US Culex pipiens (Eastern US), Culex tarsalis (Midwest and West), and Culex quinquefasciatus (Southeast) are the main sources.[12]
In mammals the virus does not multiply as readily (i.e. does not develop high viremia during infection), and it is believed that mosquitoes biting infected mammals do not ingest sufficient virus to become infected,[13] making mammals so-called dead-end infections.
A 2004 paper in Science found that Culex pipiens mosquitoes existed in two populations in Europe, one which bites birds and one which bites humans. In North America 40% of Culex pipiens were found to be hybrids of the two types which bite both birds and humans, providing a vector for WNV. This is argued to provide an explanation of why the West Nile disease has spread more quickly in North America than Europe.[14] However, these conclusions have been disputed.[15] In 2010 it was verified by the Greek Center for Disease Control and Prevention that Culex pipiens was responsible for an outbreak of the virus in northern Greece.[16]
Susceptibility
It was initially believed that direct human-to-human transmission was only caused by occupational exposure,[17] or conjunctival exposure to infected blood.[18] The US outbreak revealed novel transmission methods, through blood transfusion,[19] organ transplant,[20] intrauterine exposure,[21] and breast feeding.[22] Since 2003, blood banks in the US routinely screen for the virus amongst their donors.[23] As a precautionary measure, the UK's National Blood Service initially ran a test for this disease in donors who donate within 28 days of a visit to the United States, Canada or the North Eastern provinces of Italy. Currently (July 2010) the policy is to ask you to wait 28 days after returning from North America or the North Eastern provinces of Italy before donating.
The more severe outcomes of WNV infection are clearly associated with advancing age[24] and a patient history of organ transplantation[25] and diabetes. A genetic factor also appears to increase susceptibility to West Nile disease. A mutation of the gene CCR5 gives some protection against HIV but leads to more serious complications of WNV infection. Carriers of two mutated copies of CCR5 made up 4 to 4.5% of a sample of West Nile disease sufferers while the incidence of the gene in the general population is only 1%.[26][27]
Recently, the potential for mosquito saliva to impact the course of WNV disease was demonstrated.[28][29][30] Mosquitoes inoculate their saliva into the skin while obtaining blood. Mosquito saliva is a pharmacologic cocktail of secreted molecules, principally proteins, that can affect vascular constriction, blood coagulation, platelet aggregation, inflammation, and immunity. It has become clear that mosquito saliva alters the immune response in a manner that may be advantageous to a virus.[31][32][33][34] Studies have shown that it can specifically modulate the immune response during early virus infection,[35] and mosquito feeding can exacerbate WNV infection leading to higher viremia and more severe forms of disease.[28][29][30] It is unknown what benefit, if any, the mosquito receives by assisting the virus in this manner, so it is likely that the virus is simply exploiting the preexisting qualities of mosquito saliva developed for other purposes.
There is no vaccine for humans. A vaccine for horses (ATCvet code: QI05AA10) based on killed viruses exists; some zoos have given this vaccine to their birds, although its effectiveness there is unknown. Dogs and cats show few if any signs of infection. There have been no known cases of direct canine-human or feline-human transmission; although these pets can become infected, it is unlikely that they are in turn capable of infecting native mosquitoes and thus continuing the disease cycle.[36]
Avoiding mosquito bites is the most straightforward means to avoid infection[37]—remaining indoors (while preventing mosquitoes from entering) at dawn and dusk, wear light-colored clothing that covers arms and legs as well as trunk, use insect repellents on both skin and clothing (such as DEET, picaradin, or oil of lemon eucalyptus for skin and permethrin for clothes).[38] If one becomes infected, generally, treatment is purely supportive: analgesia for the pain of neurologic diseases; rehydration for nausea, vomiting, or diarrhea; encephalitis may also require airway protection and seizure management.
Reported cases in the U.S. in 2005 exceeded those in 2004, and cases in 2006 exceeded 2005's totals. On August 19, 2006, the LA Times reported that the expected incidence rate of WNV was dropping as the local population becomes exposed to the virus. "In countries like Egypt and Uganda, where West Nile was first detected, people became fully immune to the virus by the time they reached adulthood", federal health officials said.[39] However, just days later, the CDC said that WNV cases could reach a three-year high because hot temperatures had allowed a larger brood of mosquitoes.[40]
History
Studies of phylogenetic lineages have determined that WNV emerged as a distinct virus around 1000 years ago.[41] This initial virus developed into two distinct lineages, Lineage 1 and its multiple profiles is the source of the epidemic transmission in Africa and throughout the world. Lineage 2 was considered an Africa zoonose. However, in 2008, lineage 2, previously only seen in horses in sub-Saharan Africa and Madagascar, began to appear in horses in Europe, where the first known outbreak affected 18 animals in Hungary in 2008.[42]
WNV has been posited as one of the possible causes of Alexander the Great's early death based on reports of avian deaths before his illness period.[43]
WNV was first isolated from a feverish 37 year old woman at Omogo in the West Nile District of Uganda in 1937 during research on yellow fever virus.[44] A series of serosurveys in 1939 in central Africa found anti-WNV positive results ranging from 1.4% (Congo) to 46.4% (White Nile region, Sudan). It was subsequently identified in Egypt (1942) and India (1953), a 1950 serosurvey in Egypt found 90% of those over 40 years in age had WNV antibodies. The ecology was characterized in 1953 with studies in Egypt[45] and Israel.[46] The virus became recognized as a cause of severe human meningoencephalitis in elderly patients during an outbreak in Israel in 1957. The disease was first noted in horses in Egypt and France in the early 1960s and found to be widespread in southern Europe, southwest Asia and Australia.
The first appearance of WNV in the Western hemisphere was in 1999 with encephalitis reported in humans, dogs, cats, and horses, and the subsequent spread in the United States may be an important milestone in the evolving history of this virus. The American outbreak began in the New York City area (specifically, College Point, Queens) and was later seen in New Jersey and Connecticut; the virus is believed to have entered in an infected bird or mosquito, although there is no clear evidence.[47] The US virus was very closely related to a lineage 1 strain found in Israel in 1998. Since the first North American cases in 1999, the virus has been reported throughout the United States, Canada, Mexico, the Caribbean and Central America. There have been human cases and horse cases, and many birds are infected. The Barbary Macaque, Macaca sylvanus was the first non-human primate to contract WNV.[48] Both the US and Israeli strains are marked by high mortality rates in infected avian populations, the presence of dead birds—especially corvidae—can be an early indicator of the arrival of the virus.
A high level of media coverage through 2001/2002 raised public awareness of WNV. This coverage was most likely the result of successive appearances of the virus in new areas, and had the unintended effect of increasing funding for research on this virus and related arthropod-borne viruses. Such research has expanded our understanding of viruses transmitted by mosquitoes.
Overwintering mechanism
Vertical transmission of West Nile Virus from female Culex pipiens mosquitoes to their progeny has been demonstrated in the laboratory. It has been not suggested that vertically infected Culex could survive the winter to initiate a WNV amplification cycle the following spring. Culex mosquitoes spend the winter hibernating in protected structures such as root cellars, bank barns, caves, abandoned tunnels and other subterranean locations. The first overwintering adult mosquitoes to test positive for WNV were collected in New York, 2000. Since then, positive samples have been identified in New Jersey, 2003 and in Pennsylvania, 2003, 2004 and 2005.[49]
Geographic distribution
West Nile virus has been described in Africa, Europe, the Middle East, west and central Asia, Oceania (subtype Kunjin), and most recently, North America.
Recent outbreaks of West Nile virus encephalitis in humans have occurred in Algeria (1994), Romania (1996 to 1997), the Czech Republic (1997), Congo (1998), Russia (1999), the United States (1999 to 2009), Canada (1999–2007), Israel (2000) and Greece (2010).
Epizootics of disease in horses occurred in Morocco (1996), Italy (1998), the United States (1999 to 2001), and France (2000). In 2003, West Nile virus was found in horses in Mexico.
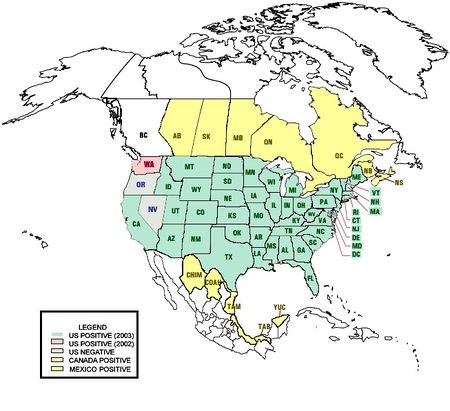
In the US in 2008, West Nile virus was reported in animals in 47 states, D.C. and Puerto Rico. 45 states and D.C. reported human cases in 2008 with only Maine, Alaska and Hawaii having never had a human case. (Maine has had occasional animal cases.)[50]
Recent outbreaks
United States: From 1999 through 2001, the CDC confirmed 149 West Nile virus infections, including 18 deaths. In 2002, a total of 4,156 cases were reported, including 284 fatalities. 13 cases in 2002 were contracted through blood transfusion. The cost of WNV-related health care in 2002 was estimated at $200 million. The first human West Nile disease in 2003 occurred in June and one West Nile-infected blood transfusion was also identified that month. In the 2003 outbreak, 9,862 cases and 264 deaths were reported by the CDC. At least 30% of those cases were considered severe involving meningitis or encephalitis. In 2004, there were only 2,539 reported cases and 100 deaths. In 2005, there was a slight increase in the number of cases, with 3,000 cases and 119 deaths reported. 2006 saw another increase, with 4,269 cases and 177 deaths. In 2007, the number of cases reported decreased to 3,623 and the number of deaths dropped to 124. In 2007, 1,227 cases of wnv neuroinvasion disease and 117 deaths occurred. In 2008, West Nile surveillance data reported to CDC, a total of 28 states have reported 236 cases of human WNV illness. A total of 137 cases for which such data were available occurred in males, median age patients was 48 years. Dates of illness onset ranged from January 17 to August 14: Two cases were fatal.

See also Progress of the West Nile virus in the United States
Canada: One human death occurred in 1999. In 2002, ten human deaths out of 416 confirmed and probable cases were reported by Canadian health officials. In 2003, 14 deaths and 1,494 confirmed and probable cases were reported. Cases were reported in 2003 in Nova Scotia, Quebec, Ontario, Manitoba, Saskatchewan, Alberta, British Columbia, and the Yukon. In 2004, only 26 cases were reported and two deaths; however, 2005 saw 239 cases and 12 deaths. By October 28, 2006, 127 cases and no deaths had been reported. One case was asymptomatic and only discovered through a blood donation. In 2007, 445 Manitobans had confirmed cases of WNV and two people died with a third unconfirmed but suspected.[51] 17 people have either tested positive or are suspected of having the virus in Saskatchewan, and only one person has tested positive in Alberta.[52] Saskatchewan has reported 826 cases of WNV plus three deaths.[53] The spread of West Nile Virus infected mosquitoes to British Columbia for the first time was reported in 2009[54]
Israel: In 2000, the CDC found that there were 417 confirmed cases with 326 hospitalizations. 33 of these people died. The main clinical presentations were encephalitis (57.9%), febrile disease (24.4%), and meningitis (15.9%).[55]
Romania: In 1996–1997 about 500 cases occurred in Romania with a fatality rate of nearly 10%.
Greece: In the summer of 2010 several cases were reported in northern Greece. As of 27 August 2010 there were 134 diagnosed cases and 9 fatalities.[16][56]
Surveillance methods
West Nile virus can be sampled from the environment by the pooling of trapped mosquitoes, testing avian blood samples drawn from wild birds and dogs and sentinel monkeys, as well as testing brains of dead birds found by various animal control agencies and the public. Testing of the mosquito samples requires the use of RT-PCR to directly amplify and show the presence of virus in the submitted samples. When using the blood sera of wild bird and sentinel chickens, samples must be tested for the presence of WNV antibodies by use of immunohistochemistry (IHC)[57] or Enzyme-Linked Immunosorbent Assay (ELISA).[58]
Dead birds, after necropsy, have their various tissues tested for virus by either RT-PCR or immunohistochemistry, where virus shows up as brown stained tissue because of a substrate-enzyme reaction.
Control

West Nile control is achieved through mosquito control, by elimination of mosquito breeding sites, larviciding active breeding areas and encouraging personal use of mosquito repellents. The public is also encouraged to spend less time outdoors, wear long covering clothing, apply bug repellant that contains DEET and ensure that mosquitoes cannot enter buildings.[59] Environmentalists have condemned attempts to control the transmitting mosquitoes by spraying pesticide, saying that the detrimental health effects of spraying outweigh the relatively few lives which may be saved, and that there are more environmentally friendly ways of controlling mosquitoes. They also question the effectiveness of insecticide spraying, as they believe mosquitoes that are resting or flying above the level of spraying will not be killed; the most common vector in the northeastern U.S., Culex pipiens, is a canopy feeder.
The first effective horse vaccine, West Nile-INNOVATOR™ was introduced by Fort Dodge Animal Health (Wyeth). Shortly thereafter, A second, one-annual-dose vaccine called Prevenile™ was introduced by Intervet/Schering-Plough Animal Health (Merck),[60] followed by a DNA-based vaccine, called Recombitek™ (Merial). In 2009, a new killed virus vaccine was introduced by Boehringer-Ingelheim, a privately held pharmaceutical company, incorporating an equine origin WNV strain (E159), representative of the more recent WNV strains impacting horses.[61]
Treatment research
AMD3100, which had been proposed as an antiretroviral drug for HIV, has shown promise against West Nile encephalitis. Morpholino antisense oligos conjugated to cell penetrating peptides have been shown to partially protect mice from WNV disease.[62] There have also been attempts to treat infections using ribavirin, intravenous immunoglobulin, or alpha interferon.[63] GenoMed, a U.S. biotech company, has found that blocking angiotensin II can treat the "cytokine storm" of West Nile virus encephalitis as well as other viruses.[64]
In 2007 the World Community Grid launched the Discovering Dengue Drugs – Together project. This uses a distributed network of volunteers' computers via the Berkeley Open Infrastructure for Network Computing (BOINC) to perform computer simulations of interacting molecules. Thousands of small molecules are screened for potential anti-viral properties with respect to West Nile and related viruses.
See also
- CCR5
- Psorophora howardii
Notes
- ↑ "Louisiana Department of Health & Hospitals". www.dhh.louisiana.gov. http://www.dhh.louisiana.gov/news.asp?Detail=1663. Retrieved 2010-08-06.
- ↑ Olejnik E (1952). "Infectious adenitis transmitted by Culex molestus". Bull Res Counc Isr 2: 210–1.
- ↑ Smithburn KC, Jacobs HR (1942). "Neutralization-tests against neurotropic viruses with sera collected in central Africa". Journal of Immunology 44: 923.
- ↑ Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI (1998). "West Nile encephalitis epidemic in southeastern Romania". Lancet 352 (9130): 767–71. doi:10.1016/S0140-6736(98)03538-7. PMID 9737281. http://linkinghub.elsevier.com/retrieve/pii/S0140673698035387.
- ↑ Sejvar JJ, Haddad MB, Tierney BC, et al. (2003). "Neurologic manifestations and outcome of West Nile virus infection". JAMA 290 (4): 511–5. doi:10.1001/jama.290.4.511. PMID 12876094.
- ↑ Ahmed S, Libman R, Wesson K, Ahmed F, Einberg K (2000). "Guillain-Barré syndrome: An unusual presentation of West Nile virus infection". Neurology 55 (1): 144–6. PMID 10891928. http://www.neurology.org/cgi/pmidlookup?view=long&pmid=10891928.
- ↑ Abroug F, Ouanes-Besbes L, Letaief M, et al. (2006). "A cluster study of predictors of severe West Nile virus infection". Mayo Clin. Proc. 81 (1): 12–6. doi:10.4065/81.1.12. PMID 16438473.
- ↑ Perelman A, Stern J (1974). "Acute pancreatitis in West Nile Fever". Am. J. Trop. Med. Hyg. 23 (6): 1150–2. PMID 4429184. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=4429184.
- ↑ Omalu BI, Shakir AA, Wang G, Lipkin WI, Wiley CA (2003). "Fatal fulminant pan-meningo-polioencephalitis due to West Nile virus". Brain Pathol. 13 (4): 465–72. PMID 14655752.
- ↑ Mathiot CC, Georges AJ, Deubel V (1990). "Comparative analysis of West Nile virus strains isolated from human and animal hosts using monoclonal antibodies and cDNA restriction digest profiles". Res. Virol. 141 (5): 533–43. doi:10.1016/0923-2516(90)90085-W. PMID 1703658.
- ↑ http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm
- ↑ Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL (2005). "Epidemiology and transmission dynamics of West Nile virus disease". Emerging Infect. Dis. 11 (8): 1167–73. PMID 16102302. http://www.cdc.gov/ncidod/EID/vol11no08/05-0289a.htm.
- ↑ Taylor RM, Hurlbut HS, Dressler HR, Spangler EW, Thrasher D (1953). "Isolation of West Nile virus from Culex mosquitoes". J Egypt Med Assoc 36 (3): 199–208. PMID 13084817.
- ↑ Fonseca DM, et al. (March 2004). "Emerging vectors in the Culex pipiens complex". Science 303 (5663): 1535–8. doi:10.1126/science.1094247. PMID 15001783.
- ↑ Spielman A, et al. (November 2004). "Outbreak of West Nile Virus in North America". Science 306 (5701): 1473–5. doi:10.1126/science.306.5701.1473c. PMID 15567836.
- ↑ 16.0 16.1 "Virus culprit tracked down". Kathimerini. 2010-08-21. http://www.ekathimerini.com/4dcgi/_w_articles_politics_2_21/08/2010_119182. Retrieved 2010-08-21.
- ↑ Centers for Disease Control and Prevention (CDC) (2002). "Laboratory-acquired West Nile virus infections—United States, 2002". MMWR Morb. Mortal. Wkly. Rep. 51 (50): 1133–5. PMID 12537288.
- ↑ Fonseca K, Prince GD, Bratvold J, et al. (2005). "West Nile virus infection and conjunctival exposure". Emerging Infect. Dis. 11 (10): 1648–9. PMID 16355512.
- ↑ Centers for Disease Control and Prevention (CDC) (2002). "Investigation of blood transfusion recipients with West Nile virus infections". MMWR Morb. Mortal. Wkly. Rep. 51 (36): 823. PMID 12269472.
- ↑ Centers for Disease Control and Prevention (CDC) (2002). "West Nile virus infection in organ donor and transplant recipients—Georgia and Florida, 2002". MMWR Morb. Mortal. Wkly. Rep. 51 (35): 790. PMID 12227442.
- ↑ Centers for Disease Control and Prevention (CDC) (2002). "Intrauterine West Nile virus infection—New York, 2002". MMWR Morb. Mortal. Wkly. Rep. 51 (50): 1135–6. PMID 12537289.
- ↑ Centers for Disease Control and Prevention (CDC) (2002). "Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002". MMWR Morb. Mortal. Wkly. Rep. 51 (39): 877–8. PMID 12375687.
- ↑ Centers for Disease Control and Prevention (CDC) (2003). "Detection of West Nile virus in blood donations—United States, 2003". MMWR Morb. Mortal. Wkly. Rep. 52 (32): 769–72. PMID 12917583. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5232a3.htm.
- ↑ Panthier R, Hannoun C, Beytout D, Mouchet J (1968). "[Epidemiology of West Nile virus. Study of a center in Camargue.]" (in French). Ann Inst Pasteur (Paris) 115 (3): 435–45. PMID 5711530.
- ↑ Kumar D, Drebot MA, Wong SJ, et al. (2004). "A seroprevalence study of west nile virus infection in solid organ transplant recipients". Am. J. Transplant. 4 (11): 1883–8. doi:10.1111/j.1600-6143.2004.00592.x. PMID 15476490.
- ↑ Glass, WG; Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM (October 17 2005). "Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection". Journal of Experimental Medicine 202 (8): 1087–98. doi:10.1084/jem.20042530. PMID 16230476.
- ↑ Glass, WG; McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM (January 23 2006). "CCR5 deficiency increases risk of symptomatic West Nile virus infection". Journal of Experimental Medicine 203 (1): 35–40. doi:10.1084/jem.20051970. PMID 16418398.
- ↑ 28.0 28.1 Schneider BS, McGee CE, Jordan JM, Stevenson HL, Soong L, Higgs S (2007). "Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted west nile virus infection". PLoS ONE 2 (11): e1171. doi:10.1371/journal.pone.0001171. PMID 18000543. PMC 2048662. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0001171.
- ↑ 29.0 29.1 Styer LM, Bernard KA, Kramer LD (2006). "Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose". Am. J. Trop. Med. Hyg. 75 (2): 337–45. PMID 16896145. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=16896145.
- ↑ 30.0 30.1 Schneider BS, Soong L, Girard YA, Campbell G, Mason P, Higgs S (2006). "Potentiation of West Nile encephalitis by mosquito feeding". Viral Immunol. 19 (1): 74–82. doi:10.1089/vim.2006.19.74. PMID 16553552.
- ↑ Wasserman HA, Singh S, Champagne DE (2004). "Saliva of the Yellow Fever mosquito, Aedes aegypti, modulates murine lymphocyte function". Parasite Immunol. 26 (6–7): 295–306. doi:10.1111/j.0141-9838.2004.00712.x. PMID 15541033. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0141-9838&date=2004&volume=26&issue=6-7&spage=295.
- ↑ Limesand KH, Higgs S, Pearson LD, Beaty BJ (2003). "Effect of mosquito salivary gland treatment on vesicular stomatitis New Jersey virus replication and interferon alpha/beta expression in vitro". J. Med. Entomol. 40 (2): 199–205. doi:10.1603/0022-2585-40.2.199. PMID 12693849.
- ↑ Wanasen N, Nussenzveig RH, Champagne DE, Soong L, Higgs S (2004). "Differential modulation of murine host immune response by salivary gland extracts from the mosquitoes Aedes aegypti and Culex quinquefasciatus". Med. Vet. Entomol. 18 (2): 191–9. doi:10.1111/j.1365-2915.2004.00498.x. PMID 15189245. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0269-283X&date=2004&volume=18&issue=2&spage=191.
- ↑ Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR (1999). "Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice". Parasite Immunol. 21 (1): 35–44. doi:10.1046/j.1365-3024.1999.00199.x. PMID 10081770. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0141-9838&date=1999&volume=21&issue=1&spage=35.
- ↑ Schneider BS, Soong L, Zeidner NS, Higgs S (2004). "Aedes aegypti salivary gland extracts modulate anti-viral and TH1/TH2 cytokine responses to sindbis virus infection". Viral Immunol. 17 (4): 565–73. doi:10.1089/vim.2004.17.565. PMID 15671753.
- ↑ CDC
- ↑ Hayes EB, Gubler DJ (2006). "West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States". Annu. Rev. Med. 57: 181–94. doi:10.1146/annurev.med.57.121304.131418. PMID 16409144.
- ↑ Fradin MS, Day JF (2002). "Comparative efficacy of insect repellents against mosquito bites". N. Engl. J. Med. 347 (1): 13–8. doi:10.1056/NEJMoa011699. PMID 12097535.
- ↑ CDC
- ↑ Yahoo
- ↑ Galli M, Bernini F, Zehender G (July 2004). "Alexander the Great and West Nile virus encephalitis". Emerging Infect. Dis. 10 (7): 1330–2; author reply 1332–3. PMID 15338540.
- ↑ West, Christy (2010-02-08). Different West Nile Virus Genetic Lineage Evolving?. The Horse, online edition. http://www.thehorse.com/ViewArticle.aspx?ID=15779. Retrieved 2010-02-10.
- ↑ "Alexander the Great and West Nile Virus Encephalitis". Centers for Disease Control and Prevention. Retrieved on 2009-04-26
- ↑ Smithburn KC, Hughes TP, Burke AW, Paul JH (June 1940). "A Neurotropic Virus Isolated from the Blood of a Native of Uganda". Am. J. Trop. Med. 20 (1): 471–92.
- ↑ Work TH, Hurlbut HS, Taylor RM (1953). "Isolation of West Nile virus from hooded crow and rock pigeon in the Nile delta". Proc. Soc. Exp. Biol. Med. 84 (3): 719–22. PMID 13134268.
- ↑ Bernkopf H, Levine S, Nerson R (1953). "Isolation of West Nile virus in Israel". J. Infect. Dis. 93 (3): 207–18. PMID 13109233.
- ↑ Calisher CH (2000). "West Nile virus in the New World: appearance, persistence, and adaptation to a new econiche—an opportunity taken". Viral Immunol. 13 (4): 411–4. doi:10.1089/vim.2000.13.411. PMID 11192287.
- ↑ C. Michael Hogan. 2008. Barbary Macaque: Macaca sylvanus, GlobalTwitcher.com
- ↑ Bugbee, LM; Forte LR (September 2004). "The discovery of West Nile virus in overwintering Culex pipiens (Diptera: Culicidae) mosquitoes in Lehigh County, Pennsylvania". Journal of the American Mosquito Control Association 20 (3): 326–7. PMID 15532939.
- ↑ 2008 Final West Nile Activity Map, Centers for Disease Control and Prevention
- ↑ Province of Manitoba | Manitoba Health | West Nile virus
- ↑ Error
- ↑ CTV.ca | Sask. reports 339 cases of West Nile, one death
- ↑ News | West Nile virus found in BC mosquitoes
- ↑ Chowers, MY; Lang R, Nassar F, Ben-David D, Giladi M, Rubinshtein E, Itzhaki A, Mishal J, Siegman-Igra Y, Kitzes R, Pick N, Landau Z, Wolf D, Bin H, Mendelson E, Pitlik SD, Weinberger M (Jul–Aug 2001). "Clinical characteristics of the West Nile fever outbreak, Israel, 2000". Emerging Infectious Diseases 7 (4): 675–8. doi:10.3201/eid0704.010414. PMID 11585531. PMC 2631759. http://www.cdc.gov/ncidod/eid/vol7no4/chowers.htm. Retrieved 2006-06-07.
- ↑ Θεσσαλονίκη: 134 τα κρούσματα του ιού του Δυτικού Νείλου (in Greek)
- ↑ Jozan, M; Evans R, McLean R, Hall R, Tangredi B, Reed L, Scott J (Fall 2003). "Detection of West Nile virus infection in birds in the United States by blocking ELISA and immunohistochemistry". Vector-borne and Zoonotic Diseases 3 (3): 99–110. doi:10.1089/153036603768395799. PMID 14511579.
- ↑ Hall, RA; Broom AK, Hartnett AC, Howard MJ, Mackenzie JS (February 1995). "Immunodominant epitopes on the NS1 protein of MVE and KUN viruses serve as targets for a blocking ELISA to detect virus-specific antibodies in sentinel animal serum". Journal of Virological Methods 51 (2–3): 201–10. doi:10.1016/0166-0934(94)00105-P. PMID 7738140.
- ↑ "Workplace Precautions Against West Nile Virus". Safety and Health Information Bulletins (SHIBs). U. S. Department of Labor Occupational Safety and Health Administration. http://www.osha.gov/dts/shib/shib082903b.html. Retrieved 2007-11-21.
- ↑ Fact sheet (2009-11-23). West Nile Virus. Blood-Horse Publications. http://www.thehorse.com/pdf/factsheets/west-nile-virus/wnv.pdf. Retrieved 2010-02-10.
- ↑ Brown, Kimberly S. (2009-11-23). New West Nile Virus Vaccines for Horses Approved. The Horse. http://www.thehorse.com/ViewArticle.aspx?ID=15334. Retrieved 2010-02-10.
- ↑ Deas, Tia S; Bennett CJ, Jones SA, Tilgner M, Ren P, Behr MJ, Stein DA, Iversen PL, Kramer LD, Bernard KA, Shi PY (May 2007). "In vitro resistance selection and in vivo efficacy of morpholino oligomers against West Nile virus". Antimicrob Agents Chemother 51 (7): 2470. doi:10.1128/AAC.00069-07. PMID 17485503.
- ↑ Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL (2005). "Virology, pathology, and clinical manifestations of West Nile virus disease". Emerging Infect. Dis. 11 (8): 1174–9. PMID 16102303. http://www.cdc.gov/ncidod/EID/vol11no08/05-0289b.htm.
- ↑ Moskowitz DW, Johnson FE (2004). "The central role of angiotensin I-converting enzyme in vertebrate pathophysiology". Curr Top Med Chem 4 (13): 1433–54. doi:10.2174/1568026043387818. PMID 15379656.
External links
- West Nile Virus - U.S. Centers for Disease Control and Prevention (CDC) page
- U.S. National Institute for Occupational Safety and Health (NIOSH) pages
- West Nile Virus Resource Guide—National Pesticide Information Center
- Vaccine Research Center (VRC)—Information concerning WNV vaccine research studies
- equinewestnile.com
- Canadian Case Surveillance
- West Nile Virus and Insecticides
- Petersen LR, Marfin AA (6 August 2002). "West Nile virus: a primer for the clinician". Ann. Intern. Med. 137 (3): 173–9. PMID 12160365. http://www.annals.org/cgi/pmidlookup?view=long&pmid=12160365.
- Morse, Dale; International Conference on the West Nile Virus; White, Dennis (2001). West Nile Virus: Detection, Surveillance and Control (Annals of the New York Academy of Sciences, V. 951). New York, N.Y.: New York Academy of Sciences. ISBN 1-57331-375-0. http://www.nyas.org/annals/detail.asp?annalID=24.
- Nature news article on West Nile paralysis
- CBC News Coverage of West Nile in Canada
- Hubálek Z, Halouzka J (1999). "West Nile fever—a reemerging mosquito-borne viral disease in Europe". Emerging Infect. Dis. 5 (5): 643–50. PMID 10511520. PMC 2627720. http://www.cdc.gov/ncidod/eid/vol5no5/hubalek.htm.
- West Nile Virus and Wildlife Disease
- West Nile Cases Drop as Immunities Emerge, Experts Say
- Nash D, Mostashari F, Fine A, et al. (June 2001). "The outbreak of West Nile virus infection in the New York City area in 1999". N. Engl. J. Med. 344 (24): 1807–14. doi:10.1056/NEJM200106143442401. PMID 11407341. http://content.nejm.org/cgi/content/full/344/24/1807.
- Low literacy materials in Spanish for WNV prevention
- Gene mutation turned West Nile virus into killer disease among crows
- West Nile Virus Genomes database search results from the Viral Bioinformatics Resource Center
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||